We begin with the definition of edentulism, which according to Ferro is the pathological state of absence of one or more natural dental elements. Edentulism can be partial or total (Figure 1), and according to Petersen has causal and secondary risk factors. [1],[2]
The causal risk factors are: genetic diseases such as ectodermal dysplasia (Figure 2 shows the panoramic X-ray of a 16-year-old boy with this disease), periodontal disease, carious lesions, traumatic lesions and iatrogenic lesions. [3]
Secondary risk factors, i.e. those that influence the onset of edentulism, include dietary habits, oral hygiene habits and access to dental care. [4]
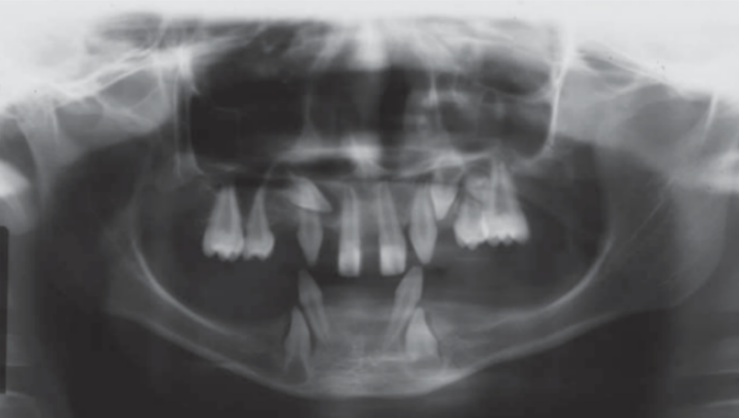
Figure 2 Panoramic x-ray of a 16-year-old boy with ectodermal dysplasia taken from Rojas, Lida Velazque, and Gisele Dalben da Silva. “Dysplasia Ectodérmica Hipohidrótica: Características Clínicas y Radiográficas”.
In order to fix the problem of edentulism, natural and non-natural elements were used since the time of the Ancient Egyptians, Chinese and later Etruscans, Hebrews and Mayans to replace missing teeth [5] ; thus, historically, a prosthesis is any artificial element (organic or inorganic) which is capable of replacing or supplementing a missing part of the human body [6] . Figure 3
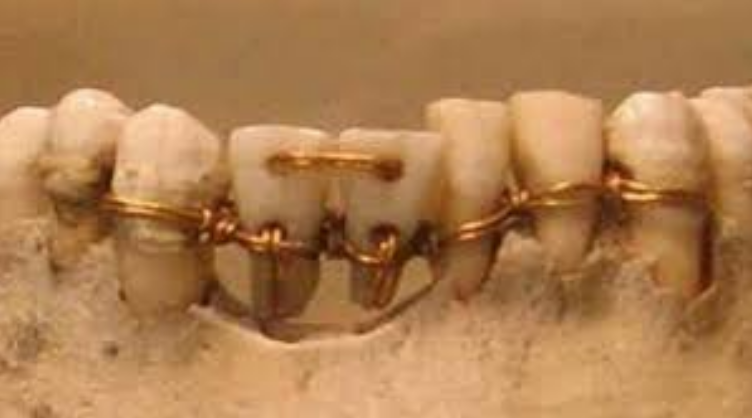
Figure 3 An Egyptian bridge made with gold thread
A prosthesis must be functional, meaning that it must restore the proper activity of a tissue, organ or system.
In the case of prostheses, they must restore the teeth and sometimes part of the bone crests, in order to restore chewing and phonatory function. [7], [8], [8], [9], [10]
Prostheses must also fit the patient's face; we therefore do not want to talk about aesthetic prosthesis but rather mimetic prosthesis [7], [11] .
Mimetic derives from the Greek μίμησις (mìmesis), meaning, “The most realistic and impersonal reproduction possible that can escape sight and observation”, and quoting Aristotle, “imitation of the ideal form and function of reality, coming as close as possible to the nature of things”.
The desirable aim of the dentist's and dental technician's work is therefore to insert a restoration that blends into the patient's body in the best possible way; because as various studies show, not only is there the influence of chewing on the cranio-cervical-mandibular system but the central nervous system is also impacted [12], [13], [14] .
As studies by De Cicco, Hirano and Sakamoto show, chewing seems to bring about an improvement in cognitive and neurodegenerative processes, since through chewing the trigeminal nerve stimulates the locus coeruleus and the reticular substance of the midbrain [15], [16], [17], [18], [19] .
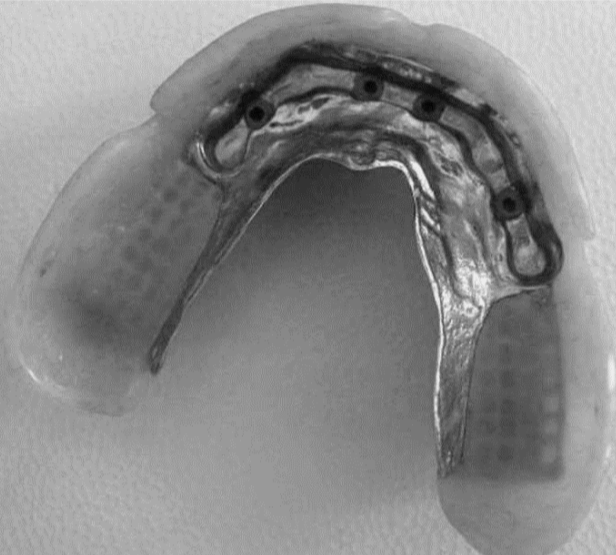
In order to achieve these outcomes, prosthetic rehabilitation consists of four different sections: the prosthetic abutment, i.e. the tooth or implant, the framework or mesostructure, e.g. the metal portion of skeletons (Figure 5), the prosthetic body, such as the pink resin section in full prostheses, and the replacement teeth [20] . In the case of a single crown, the prosthetic body and the replacement element are combined in
the crown itself. Figure 6
There are different types of prostheses: There are single crowns on teeth or implants, bridges on teeth or implants and full prostheses that rehabilitate not only the crowns but also part of the lost bone crest and are: removable partial prostheses, full prostheses, Toronto bridge hybrid prostheses and overdentures on natural teeth or implants. As the type of prosthesis varies, so do the materials which they are made from and thus their mechanical characteristics; the biological characteristics and production methods of the prostheses
themselves also vary. [21], [22], [23], [24], [25]
The various materials that make up a prosthesis are divided into metallic and non-metallic. Metallic materials include noble alloys, non-noble alloys (which are the focus of this paper), titanium alloys and aluminium alloys. Non-metallic materials are polymethylmethacrylate (PMMA), acrylic resins, ceramics, zirconia, composite materials (fibreglass, carbon fibre, composite, etc.)
Any type of prosthesis can give rise to complications since it is a structure that was not born and bred together with the function it is intended for, and therefore was not shaped and moulded by the function itself.
Complications with prostheses can arise due to system overload, ageing, wear and tear, corrosion, incorrect clinical manoeuvres or an inappropriate choice of material and rehabilitation solution. They are divided into biological, mechanical and technical complications. [26], [27], [28], [29], [30], [31], [32], [33]
Biological complications are: periodontitis, peri-implantitis and peri-implant mucositis, adverse reactions, oral soft tissues (tattoos, stomatitis, ulcers…), sensitivity disorders [28] .
Yi et al. (2020) demonstrated that the emergence profile of a prosthesis must have an angle of 30° as an upper limit, otherwise there is a high risk of peri-implantitis. [34], [35], [36]
Mechanical complications mainly depend on the overloading of the system and lead to loss of stability of the prosthesis. In the case of implants, loss of preload or fracture of the implant-prosthesis connection screw, decementation of the crown up to fracture of the prosthetic abutment (tooth or implant). [26], [27], [37], [38], [39], [40], [41], [42], [43]
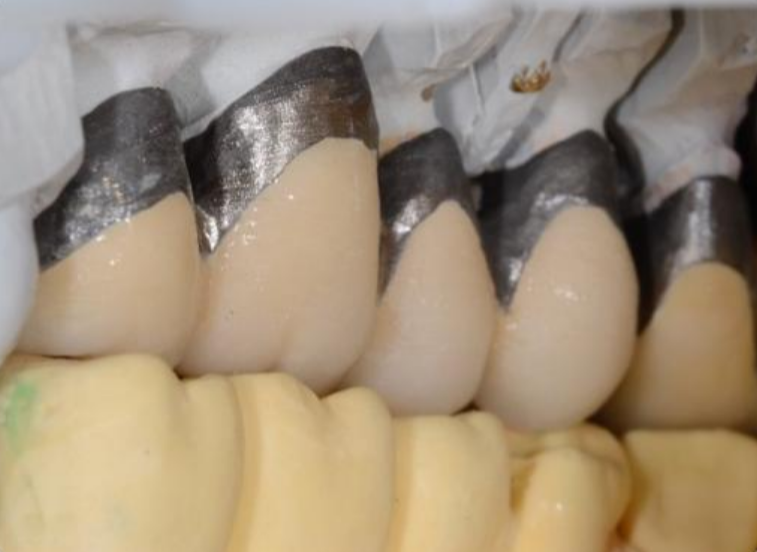
Technical complications mainly depend on the design of the prosthesis and the materials used and are: fracture of the crown or replacement elements and fracture of the framework or prosthetic body. [44], [45], [46] Figure 7
In the systematic literature review by Pjetursson et al. (2018), [29] the difference in strength and integrity between metal ceramic crowns and zirconia crowns at a 5-year follow-up was analysed.
It was observed that the 5-year success rate is about 90% for zirconia and 95% for metal ceramics.
However, Pjetursson makes a point which is that if chipping occurs, i.e. a crown is chipped, if this crown is made of metal ceramic the fracture remains limited to the ceramic portion and does not affect the stability of the prosthesis in the oral cavity; on the other hand, if chipping occurs on the zirconia, this fracture is often associated with the entire prosthetic body and results in the failure of the prosthesis. Figure 8
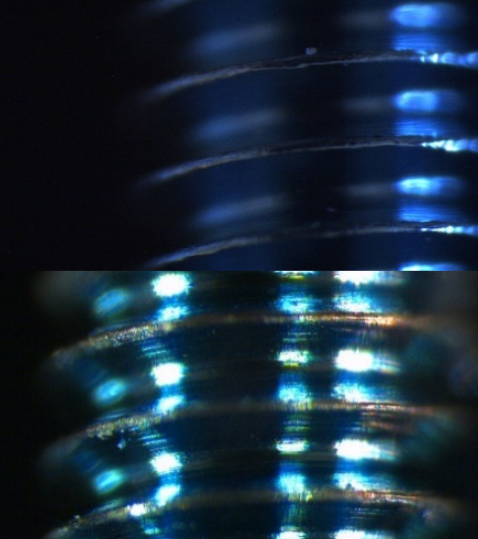
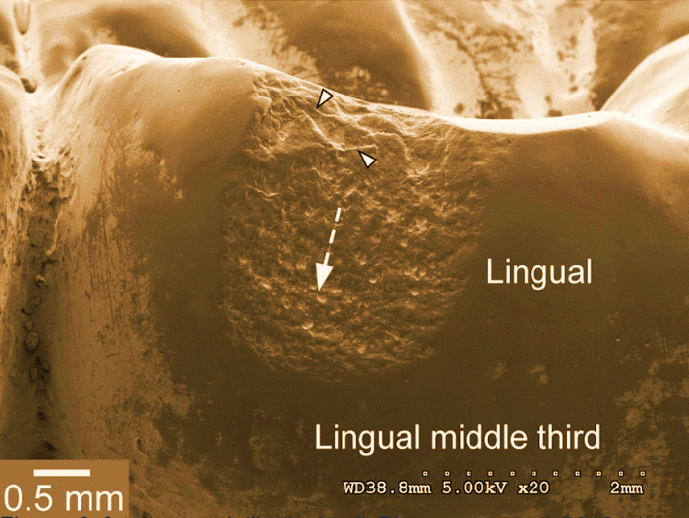
Figure 8 photo taken from the article by Pjetursson et al. (2018) showing a chipped crown under a scanning microscope
From a technical point of view, prosthetic rehabilitation must incorporate 4 fundamental parameters in order to be long-lasting.
These are:
-compliance with minimum thicknesses
-the anatomical design of the support structures
-respect for connection areas
-precision.
Compliance with the minimum thicknesses includes an all-round comparative assessment of the clinical and technical aspects; it will therefore be essential to provide a preparation of the teeth that takes into account the space of the framework and the space of the veneering material, regardless of the type of preparation being performed (vertical or with a finishing margin). Figure 9
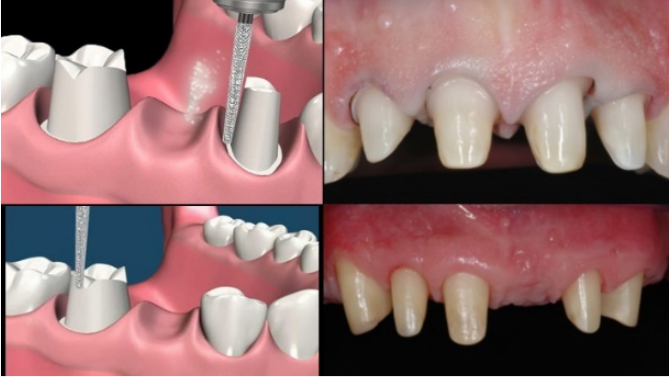
Figure 9 Horizontal or vertical finishing margin preparations
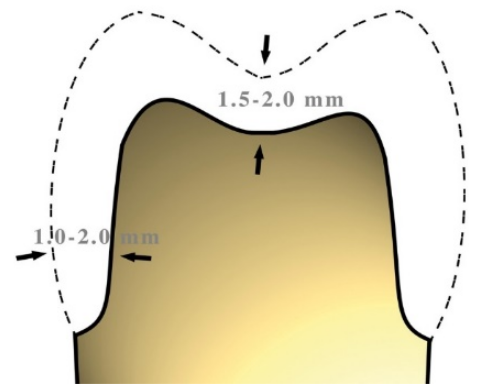
Figure 10 Minimum thicknesses for a crown
The minimum reference values for a preparation with sufficient space to ensure a good result should not be less than 1.5mm considering that the metal substructure must have a minimum thickness of 0.5mm below which it becomes risky to work. Figure 10
Supporting metal structures with a thickness of less than 0.5 mm may become deformed whilst being worked on due to a variety of factors. This could be due to the expansion and contraction movements that occur during the firing and subsequent cooling in the ceramisation phase. Figure 10
The same values must also be observed in the case of an implant framework screwed or cemented onto pre- cast stumps, as well as in the customisation of the stumps.
In the area of minimum thicknesses, heat treatment comes into play, which the metal requires to ensure stability throughout the entire workmanship process.
This treatment known as homogenisation serves to ensure that the structure's movements due to heating are neutralised before the aesthetic material is applied and before the intra-oral metal test. The heat treatment must be performed on both cast and milled metal, as well as on metal produced by laser melting, albeit for different reasons.
On the cast metal, it must be performed to re-establish the correct crystalline structure of a material that has undergone a transformation process in which the state transitions were solid/liquid and liquid/solid, resulting in a change in the crystalline structure and thus a loss of strength for the material; it is therefore essential to perform this heat treatment to restore the crystalline structure of the prosthetic framework to its origin.
For milled metal, it must be performed in order to “stretch the structure”, which during milling has incorporated stresses that could be released when the aesthetic material is being covered and thus create problems such as cracks.
It is also useful in this case to ensure that the structure is completely free from any milling oils.
Laser melting is performed to stabilise the structure obtained by laser melting metal powders. It is good to ensure that this treatment is carried out directly by the manufacturer as this stabilisation must be performed using the production plate as a base before the parts are removed.
The heat treatment for the first two cases (melting and milling) consists of bringing the mesostructure (commonly known as a framework or the metal core of the ceramic or resin veneer) to 1080°C and leaving it in atmosphere for 10 minutes, while for laser melting it is advisable to check that the treatment is carried out by the manufacturer on the framework creation plate and for a much longer time.
The anatomical design of the framework is no less important for the success and durability of a restoration with a metal substructure.
In order to create a correct anatomical structure, it is necessary to occupy the full, appropriate volumes of the finite elements, whether working in analogue (Figure 11) or digital (Figure 13).
In analogue, we will create a reduction guided by the templates that will help us devise the correct thicknesses for the aesthetic material (Figure 12).
Figure 11 Finite element analogue waxing Mr. D. Carulli
Figure 12 Finite element analogue reduction guided by silicon templates for the evaluation of minimum thicknesses. Mr. D. Carulli
Working digitally, we will perform the same process in terms of the final thicknesses to be obtained, but taking into account a very important factor: that the software performs a purely mathematical and uniform reduction of the value we require.
It is therefore essential to calculate a lower reduction value than the final one and to then adjust the correct spaces in the free-form phase without losing support where we need it. (Figure 13-14)
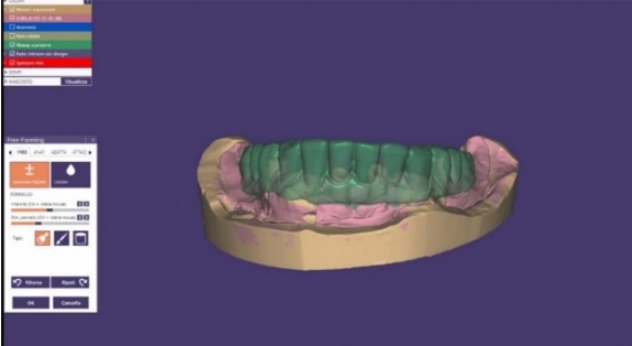
Figure 13 Waxing in a common dental cad-cam programme
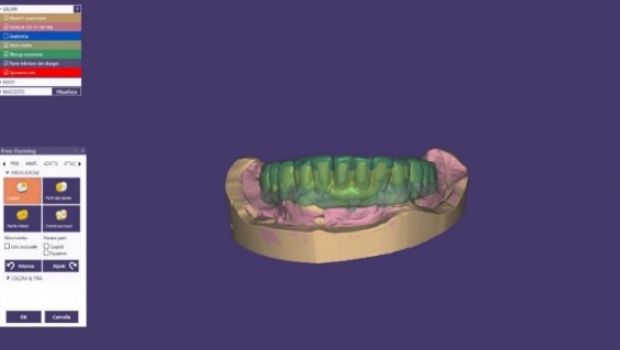
Figure 14 Adjustment of the metal framework thicknesses in the cad free-form phase

Figure 15 Example of the waxing of a framework before the lost wax casting technique. Mr. D. Carulli
Thanks to this protocol, we will be able to create milled or cast metal frameworks on natural or implant abutments that will be perfected after testing in order to optimise the spaces Figure 16
The design of the correct support structure also includes respect for the connection areas. Various factors come into play when considering which evaluations to make in order to achieve proper connection areas.
Of these we should first of all analyse the position of the natural or the implant abutments since the forces which they are subjected to are different, and particularly in the frontal areas, the forces will be mainly transverse, while in the posterior areas they will be mainly vertical.
The connectors will have to be supported more in the direction of these forces to give sufficient rigidity to the structure, although in the frontal area the horizontal thickness (depth) often does not go hand in hand with the aesthetic requirement, and the verticality of the connectors is therefore also of importance here. (Figure 17) We can thus say that the solution is the right compromise between the strength of the frame and the most satisfactory aesthetic solution.
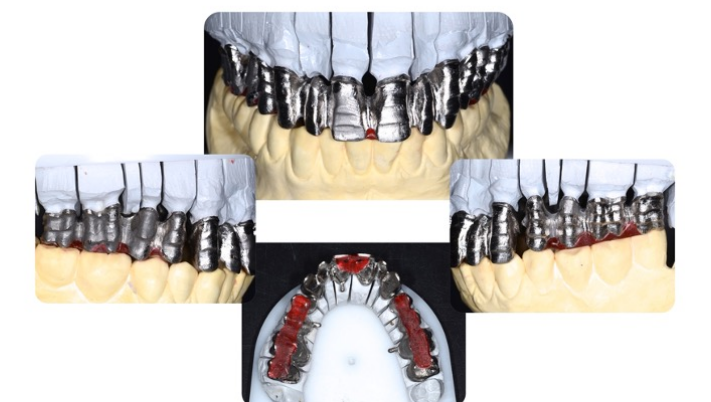
Figure 16 Respect for the connection areas Mr. D. Carulli
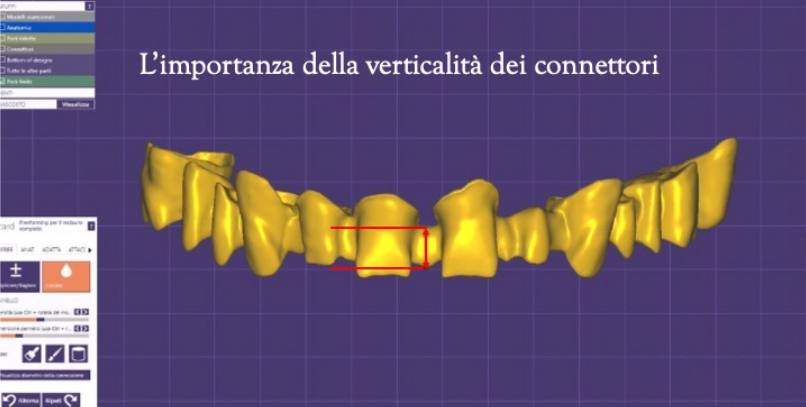
Figure 17 CAD visualisation of the connectors
Clearly, not only the direction of the major chewing forces determines the calculation of the connection areas, but also other factors such as:
-the number of abutments
-the length of the whole bridge
-the distance between the abutments
-the relationship between the abutments and middle teeth
Together, these factors make it essential to carefully evaluate the shape and total area of the connections, as well as the palatal or lingual shoulder, which is an element that contributes to the rigidity of the structure's design (fig.6)
Within this context, it is necessary to ensure that the framework is absolutely resistant to withstand the work of the aesthetic materials.
The characteristic to take into consideration regarding the metal we will be using is the value of the deformation load of the metal given by the modulus of elasticity, which is a value that indicates the rigidity of a material; between two materials subjected to the same stress, the one with a higher modulus of elasticity will undergo less deformation. This figure, usually expressed in Newton/mm² or Pascal, will help us to
choose the best alloy to use in each specific case.
Of the four parameters we have examined, precision cannot be considered the least important; on the contrary, like the others, it contributes to obtaining long-lasting prostheses. [47], [48], [49], [50]
The evolution in materials has led Mesa to obtain new-generation Cr-Co alloys nowadays that, when applied using the right protocols, thanks to their smoothness and exceptional reproduction of detail, enable results that can be superimposed onto those achievable with noble alloys. Figure 18
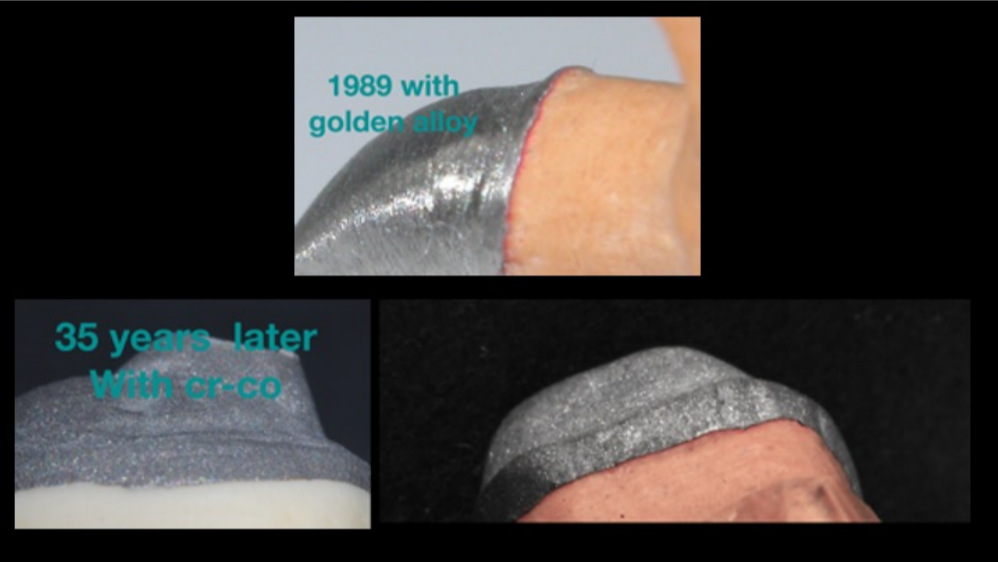
Figure 18 Precision using Mesa Cr-Co alloys Mr. D. Carulli
The arrival of digital processes to the world of dentistry and dental technology certainly offers a greater range of possibilities in metalworking than some time ago, such as milling from solid and laser melting.
The most recent research is in agreement that at present the highest marginal, axial and occlusal precision can be achieved by applying the lost wax casting method compared to the other methods that are available. [51], [52]
One of the strict protocols for dental technicians that ensures high standards of precision for prosthetic restorations consists of the following steps performed under a special stereo microscope:
1. Clean the stump with hexane or rectified spirit, checking the result under the microscope
2. Insulate with a light layer of non-alcoholic insulating fluid and blow off any excess
3. Mark the edge with a red wax pencil
4. Using an electric spatula adjusted to the melting temperature (T°) of the wax, add material by touching the stump to amalgamate the wax-wax junction, leaving a little extra outline
5. Create a smooth, even collar using a beaver tail spatula
6. Using an electric spatula set at wax softening temperature and bent into an L-shape, remove any excess from the previous extra outline
7. Clean the smears left by the electric spatula with a piece of cotton and hexane
8. A sharp blade is used at high magnification (e.g. 32x) to remove any small smears that are not visible to the naked eye
9. Apply the casting sprue, which must ALWAYS be RIGID both for single crowns and on bridges with an axis that is in the direction with the axis of deflection of the mould
10. With the help of a dam and remaining as still as possible, slide the stump under the microscope and check for the absence of wax and that the edge is intact.
The casting result is not dependent on the casting technique chosen, whether this be flame, induction or die-casting, nor on the preferred system, i.e. slow or fast, but rather it is the application of a well-defined protocol that enables us to achieve a high quality result. Figure 18
Respect for all these parameters, and the best use of materials through in-depth knowledge of them, allow us to achieve long-lasting workmanship with both natural and implant stumps.
A specific paragraph must be devoted to finishing the restorations; they must be perfectly polished, thus paying special attention to all the sub-gingival areas where bacteria spread and stagnate, as well as to corrosion due the restoration not being properly finished.
Perfect polishing in the trans-mucosal tunnel areas in implant prosthetics helps to avoid the inflammatory action of residual plaque, and a less corrosive attack, thus preserving the restoration in the best possible way and minimising the risk of bacterial adhesion and/or inflammation at the prosthesis/implant connection.
Thus, from the above, modern non-precious metal alloys allow all the available techniques to be broadly handled by both the clinician and the dental technician while maintaining high biological, functional and mimetic standards; at present, the right workmanship of non-precious metal alloys guarantees technical results that can be superimposed on noble alloys.
In non-precious metal alloys, one of the causes of the development of a complication is corrosion because in the oral cavity, given it is a moist environment in which salts are dissolved, there is the possibility of an electrochemical corrosion process occurring due to the presence of several metals (bimetallism).
The presence of fluorides and the change in pH can accelerate corrosion, which can manifest itself in the presence of metal surfaces that are no longer shiny to the point of having metal surface minuses. [53], [54], [55]
The release of ions from prosthetic material may result in an accumulation in the organs and apparatuses in other body regions far from the oral cavity; this occurrence is also assessed by means of the blood haemoglobin test. [33], [54], [54], [56]
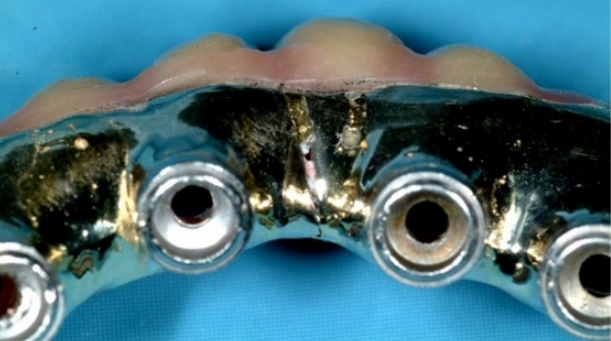
The accelerated advancement of normal prosthesis wear can be caused by corrosion phenomena that weaken the surface of the prosthesis and make the entire structure more prone to fracture. An example can be seen in the images in Figure 19-a/b shown here, where we can see the complete loss of structural integrity of a Toronto bridge due to corrosion. [57], [58], [59], [60]
Not only the framework and the prosthetic body are affected by the corrosion, but also implant abutments as can be clearly seen in Figure 20-a/b where three retained implant overdenture abutments placed on implants in the mandibular arch had their surfaces completely corroded, which also resulted in the loss of the retention head of one of the three abutments.

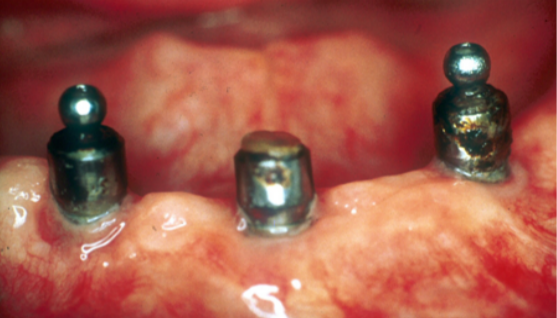
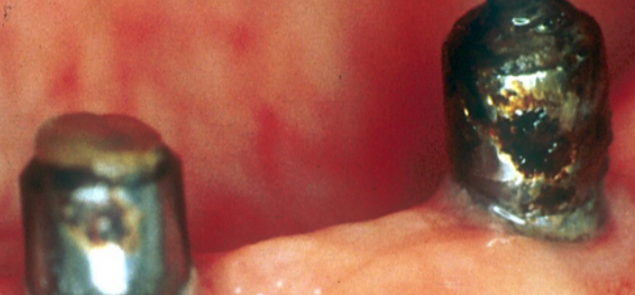
Several studies in the literature describe how the release of metal ions in the oral cavity can cause biologically adverse reactions such as gingival hypertrophy, erythema, pain or lichenoid reactions.
Undesirable systemic effects up to and including the risk of metal ion accumulation in other parts of the body are also described in the literature. [54], [58], [59], [61], [62], [63]
In the literature, undesirable effects strictly related to the oral cavity have a low incidence even in particularly susceptible individuals such as patients with nickel contact allergy, who, despite showing allergic symptoms on the epidermis, often do not experience adverse reactions to nickel in the oral cavity. [33], [59], [64]
The systematic review by Watha et al (2000) points out that in order to minimise corrosion, and thus undesirable effects, dentists should use noble or similarly noble metal alloys such as cobalt chrome. [64]
The process of electrochemical corrosion occurs when a metal is in a solution containing a low concentration of its ions; the atoms on the surface of the metal tend to pass into solution as cations (i.e. without any electrons), thus leaving electrons on the metal itself, which becomes negatively charged. This results in the formation of a “half stack”, which is improperly referred to as an “electrode”. [65] Figure 21.
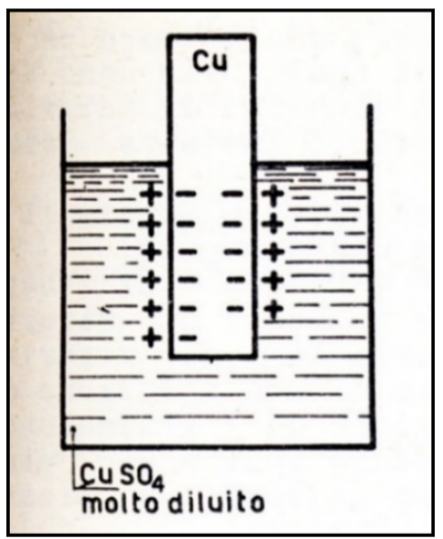
Figure 21 Schematic representation of a “half stack” or “electrode” of Copper (Cu)
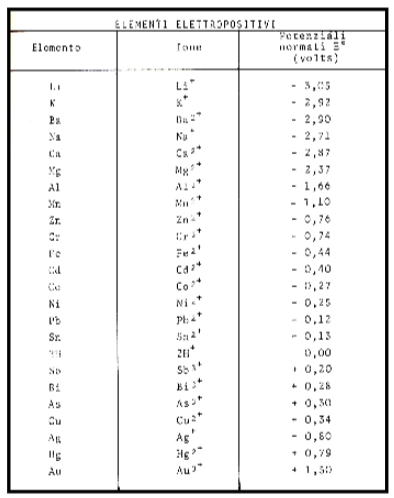
The tendency to send cations into solution for some metals is very strong (e.g. Zinc-Zn), for others it is less so (Iron-Fe; Tin-St), for others negligible (Titanium-Ti), for others almost absent (Gold-Au, Palladium-Pd, Platinum-Pt); for the latter we speak of inert metals (see Figure 22 Electrochemical series table).
Hydrogen (H), although it is not a metal, also has a tendency to release cations by transforming into H + .
Due to this tendency to send positive ions into solution, metals and hydrogen are called “electropositive” elements.
In saliva, which is an electrolyte solution, i.e. a solution containing salts, if we immerse two metals with different electropositivity, two half-stacks are formed and an electron transfer occurs from the metal with lower electropositivity (higher oxidation number) to the metal with higher electropositivity (lower oxidation number).
A “galvanic coupling” is spontaneously created in which the cathode and anode are represented by two defined zones called the microcathode and microanode.
The consequence of electron loss leads to the formation of metal cations that are released into the electrolyte solution as molecular metal, leading to corrosion of the metal acting as the anode. Figure 23
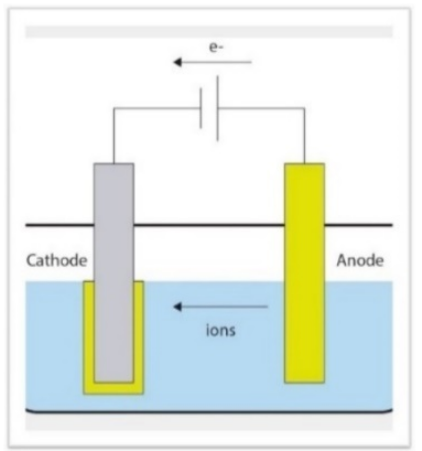
Figure 23 Example of galvanic coupling. The anode (less electropositive metal) releases cations into solution and thus corrodes. Metal ions can migrate into the systemic circulation carried by body fluids.
Following the use of Chrome-Cobalt (Cr-Co) in dentistry, several studies have evaluated the electrochemical behaviour of this material combined with other metals [33], [57], [61], [62], [63], [66], [67] .
The study by Reclaru et al.(2005) [63] shows how cobalt chrome associated with noble metals such as gold (Au) and palladium (Pd) acts as an anode and thus corrodes; the corrosion found is limited to the surface only and the consequences are clinically negligible.
In the study by Galò et al. (2012) [61] , the corrosion status of the galvanic association between Cr-CO and NiCr and between Cr-Co and Titanium gr4 and Titanium gr.5 is evaluated. In this study, it was observed that Cr-Co always acts as a cathode compared to the other elements.
So it is Ni-Cr and Titanium that corrode when combined with Cr-Co.
These results have been confirmed by a study conducted at the University of Ferrara (Ferrara, Italy) with the research group of the Department of Prosthetics and Cranio Mandibular Disorders of the Faculty of Dentistry and Dental Prosthetics led by Prof. S. Catapano on behalf of MESA Italia S.r.l. (Travagliato (Bs), Italy) at the corrosion centre of the same university directed by Prof. A. Balbo.
This study involved the 4-day combination of Cr-Co-MO and Cr-Co-W run by MESA Italia S.r.l. (Travagliato (Bs), Italy) with grade 4 titanium from the same company in 4 different artificial saliva solutions: Figure 24
nr1. Artificial saliva solution with a pH of 6.7 nr2. Artificial saliva solution with a pH of 3 nr3. Artificial saliva solution with a common fluoride mouthwash at 1000ppm nr4. Artificial saliva solution with a pH of 3 with NaF at 1000ppm With this study, it was seen that both Cr-Co-Mo and Cr-Co-W have cathodic tendencies and Titanium has an
anodic tendency, meaning that Titanium corrodes; in this electrochemical association, Titanium corrodes mainly in the first 30 hours of association.
As can be seen in the graph in Figure 25, the dissociation curve of Ti (in black), i.e. the curve indicating how many ions the metal releases in solution, starts at Time 0 at a Potential of -0.60V compared to that of Cr-Co-
W (in red) and Cr-Co-Mo (in blue) which is about -0.40V, this means that titanium corrodes i.e. releases 10 2 more cations in solution than the other 2 metals.
About 30 hours after the start of the test, the dissociation potentials of the three metals settle at -0.30V, which means that the titanium stabilises and no longer corrodes.
So it seems that after 30 hours, the surface of Titanium stabilises due to the formation of surface oxide, which isolates the surface of Titanium, making it less susceptible to corrosion.

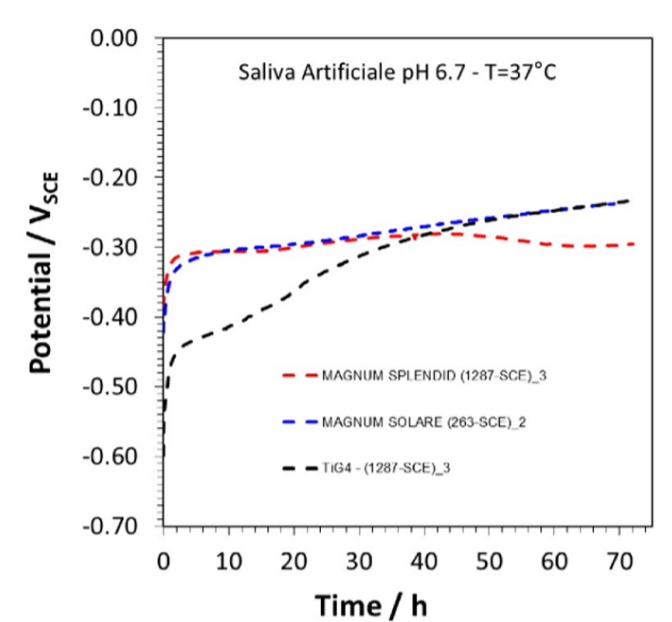
Figure 25 Dissociation graph of Cr-Co-Mo (blue), Cr-Co-W (red), Ti Gr.4 (black). From the graph, it can be seen that the Titanium curve rises and overlaps on that of the other two metals, which means that it is no longer corroding.
The pattern of galvanic associations between Cr-Co-M and Ti and between Cr-Co-W and Ti described above is superimposable both in the pH 6.7 solution (Figure 26-a) and in the pH 3 solution without NaF (Figure 26- b) and also in the solution with the mouthwash containing 1000ppm fluoride (Figure 26-c).
In the graphs below, the average of the potential differences of the three solutions during the various tests can be seen through the strongest lines and is about -0.25V.
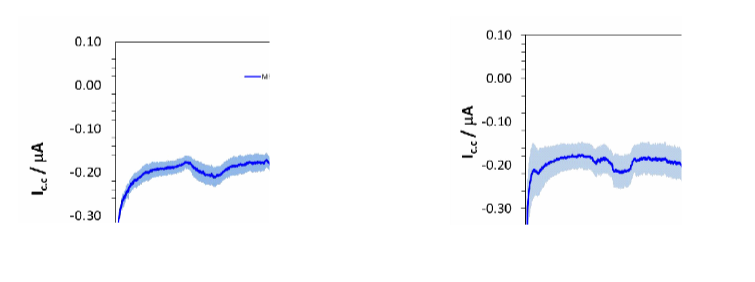
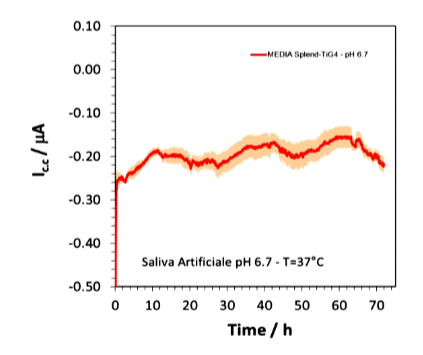
Figure 26-c Cr-Co-W and grade 4 Ti association in artificial saliva with mouthwash at a pH of 6.7
Figure 27 shows the association graph of Cr-Co-W (in red), Cr-Co-Mo (in blue) and grade 4 Ti (in black) in artificial saliva at a pH of 3 in the presence of sodium fluoride (NaF) at 1000ppm.
In this graph, the dissociation values do not change compared to the other tests for the two Cr-Co alloys as they settle at values of around -0.30V, whereas in this solution, the value of grade 4 Ti is -1.0V compared to – 0.60V in the tests described above.
This result indicates that titanium, in the solution of artificial saliva at a pH of 3 in the presence of sodium fluoride (NaF) at 1000ppm, corrodes at about 4 orders of magnitude(10 4 times ) more than other solutions.
Thus, the titanium in the latter solution (artificial saliva at a pH of 3 and NaF 1000ppm) corrodes exponentially more than in the solution with the same pH (artificial saliva at a pH of 3 without NaF ions) and 11 in the solution with the same fluoride concentration (artificial saliva and mouthwash with 1000ppm fluoride); the effect of this difference can be clearly seen by comparing Figure 28-a/b and Figure 29-a/b.
In the following images taken using a scanning microscope at 500 magnifications, the effect of corrosion on the titanium surface can be seen before (Figure 28-a) and after the test in the pH 3 solution with 0.1% NaF (Figure 28-b).
Figure 29-a shows the surface of the titanium before immersion with the mouthwash solution containing 1000ppm fluoride and Figure 29-b shows the surface of the titanium at the end of the test.
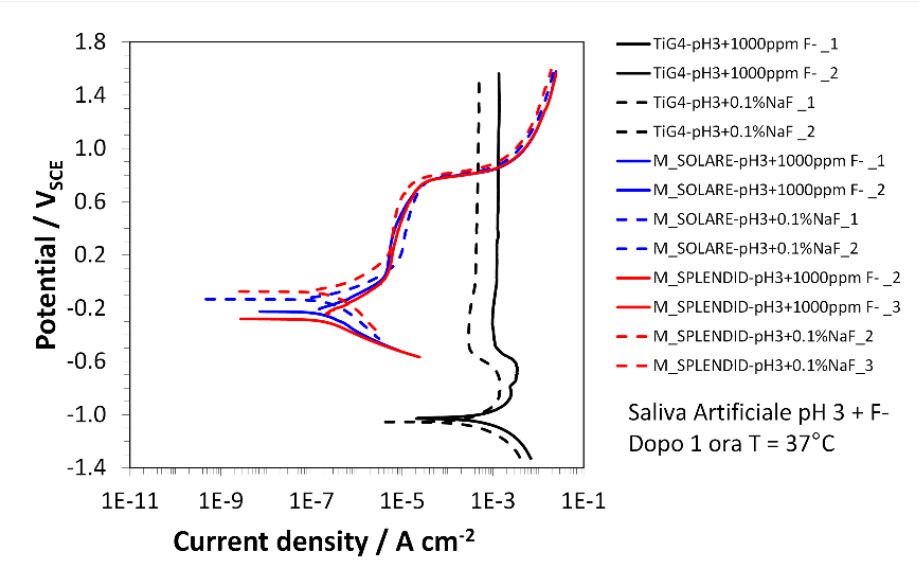
Figure 27 Dissociation graph in artificial saliva solution at a pH of 3 with NaF at 1000ppm, Cr-co-w in blue, Cr-Ci-Mo in red to grade 4 Ti in black. The values taken from this graph were from two tests, the first marked with solid lines and the second with dotted lines. The dissociation values, however, do not change for the two Cr-Co alloys, which settle at values of approximately -0.30 V while the value of the grade 4 Ti in this solution is -1.0V
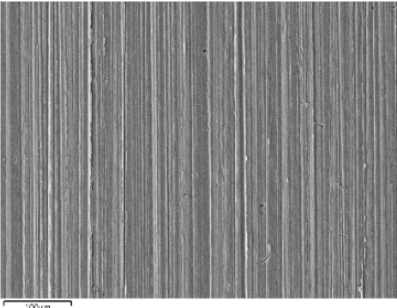
Figure 28 -a grade 4 Ti at 500x under a scanning microscope
BEFORE testing in the pH 3 artificial saliva solution with NaF at 1000ppm
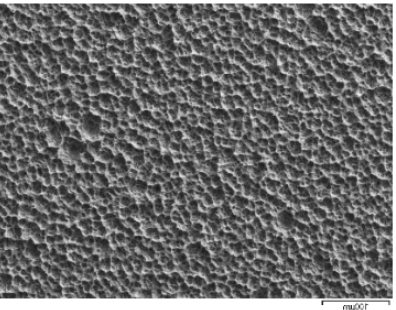
Figure 28-b grade 4 Ti at 500x under the scanning microscope
AFTER testing in the pH 3 artificial saliva solution with NaF at 1000ppm.

As can be seen from Figures 28-a and 29-a, the surface of grade 4 Ti before immersion in the various
solutions is uniform and smooth, very similar to the surface in Figure 29-b obtained after the test in the
fluoride mouthwash solution.
Similar results were obtained after tests with pH 6 and pH 3 artificial saliva without fluoride. Figure 28-b, showing the surface of titanium after testing in pH 3 artificial saliva solution with NaF at 1000ppm shows a surface that has been severely compromised by corrosion.
The image shows how, due to electrochemical corrosion, the surface of titanium has turned from smooth to rough and is completely covered with gaps of material with an average diameter of about 10µm.
These gaps represent the points on the titanium surface where cations of the metal have detached from the surface and migrated into the solution.
The exponentially different result between the solution with 1000ppm fluoride mouthwash and the pH 3 solution with 1000ppm NaF (i.e. the same fluoride concentration) can be explained by the fact that the mouthwash contains buffering substances that prevent the advancement of corrosion stimulated by the presence of fluoride. [67]
So, in summary, the electrochemical couplings of Cr-Co in the oral cavity can be:
Between Cr-Co and noble metals, Cr-Co is the anode, so the latter is the metal that corrodes.
Between Cr-Co and Ni-Cr, Cr-Co is the cathode, so the metal that corrodes is Ni-Cr.
Between Cr-Co and Ti, Cr-Co is the cathode, so Ti corrodes; but its corrosion seems to stabilise after 30h due to the surface oxides of the titanium.
In the Cr-Co and Ti metal coupling in the presence of NaF ions, Ti corrodes about 10 4 times more than under normal saliva conditions at a pH of 6.7.
The conclusions of this research are that the choice of material and type of rehabilitation technique must be adapted to the biological and functional demands of each individual case.
Modern non-precious alloys allow a broad handling of all available techniques for both the clinician and the dental technician while maintaining high biological, functional and mimetic standards; as long as the processing of non-precious alloys is correct at all stages, Cr-Co can guarantee technical results comparable to noble alloys with less framework deformability.
Electrolytic corrosion of Cr-Co in the oral cavity is clinically negligible, but it is always preferable to combine metals with similar electropositivity.
Bibliography:
[1] ‘The Glossary of Prosthodontic Terms’, The Journal of Prosthetic Dentistry, vol. 117, no. 5, pp. C1-e105, May 2017, doi: 10.1016/j.prosdent.2016.12.001.
[2] C. E. Misch, Dental implant prosthetics. St. Louis, Mo.: Elsevier, Mosby, 2015.
[3] L. V. Rojas and G. D. da Silva, ‘Displasia ectodérmica hipohidrótica: características clínicas y radiográficas’, Revista Odontológica Mexicana, vol. 19, no. 4, pp. 253–257, Oct. 2015, doi: 10.1016/j.rodmex.2015.10.007.
[4] P. E. Petersen, D. Bourgeois, H. Ogawa, S. Estupinan-Day, and C. Ndiaye, ‘The global burden of oral diseases and risks to oral health’, Bull World Health Organ, vol. 83, no. 9, pp. 661–669, Sep. 2005.
[5] P. Zampetti, Storia dell’odontoiatria, 1. ed. in Aracne] A06, [Scienze mediche, no. 110. Roma: Aracne, 2009.
[6] Peter Lerch, La protesi totale la nuova sintesi- fisiologia e funzione. 1987.
[7] M. Avrampou, R. Mericske-Stern, M. B. Blatz, and J. Katsoulis, ‘Virtual implant planning in the edentulous maxilla: criteria for decision making of prosthesis design’, Clin. Oral Impl. Res., vol. 24, pp. 152–159, Aug. 2013, doi: 10.1111/j.1600-0501.2011.02407.x.
[8] N. U. Zitzmann and C. P. Marinello, ‘Treatment plan for restoring the edentulous maxilla with implant-supported restorations: removable overdenture versus fixed partial denture design.’, The Journal of prosthetic dentistry, vol. 82, no. 2, pp. 188–196, 1999, doi: 10.1016/S0022-3913(99)70155-1.
[9] N. U. Zitzmann and C. P. Marinello, ‘Fixed or removable implant-supported restorations in the edentulous maxilla: literature review’, Pract Periodontics Aesthet Dent, vol. 12, no. 6, pp. 599–608; quiz 609, Aug. 2000.
[10] L. Zhang, C. Lyu, Z. Shang, A. Niu, and X. Liang, ‘Quality of Life of Implant-Supported Overdenture and Conventional Complete Denture in Restoring the Edentulous Mandible: A Systematic Review’, Implant Dentistry, vol. 26, no. 6, pp. 945–950, Dec. 2017, doi: 10.1097/ID.0000000000000668.
[11] S. Agarwal and S. Maiti, ‘REALITY OF LEON WILLIAM’S CONCEPT IN INDIAN SCENARIO-A RETROSPECTIVE STUDY’, 2020.
[12] M. Miçooğulları, İ. Yüksel, and S. Angın, ‘Effect of pain on cranio-cervico-mandibular function and postural stability in people with temporomandibular joint disorders’, Korean J Pain, vol. 37, no. 2, pp. 164–177, Apr. 2024, doi: 10.3344/kjp.23301.
[13] M. T. Khan, S. K. Verma, S. Maheshwari, S. N. Zahid, and P. K. Chaudhary, ‘Neuromuscular dentistry: Occlusal diseases and posture’, J Oral Biol Craniofac Res, vol. 3, no. 3, pp. 146–150, 2013, doi: 10.1016/j.jobcr.2013.03.003.
[14] C. D. Westersund, J. Scholten, and R. J. Turner, ‘Relationship between craniocervical orientation and center of force of occlusion in adults’, Cranio, vol. 35, no. 5, pp. 283–289, Sep. 2017, doi: 10.1080/08869634.2016.1235254.
[15] V. De Cicco, M. Barresi, M. P. Tramonti Fantozzi, E. Cataldo, V. Parisi, and D. Manzoni, ‘Oral Implant-Prostheses: New Teeth for a Brighter Brain’, PLoS ONE, vol. 11, no. 2, p. e0148715, Feb. 2016, doi: 10.1371/journal.pone.0148715.
[16] Y. Hirano and M. Onozuka, ‘[Chewing and cognitive function]’, Brain Nerve, vol. 66, no. 1, pp. 25–32, Jan. 2014.
[17] K. Sakamoto, H. Nakata, and R. Kakigi, ‘The effect of mastication on human cognitive processing: a study using event-related potentials’, Clin Neurophysiol, vol. 120, no. 1, pp. 41–50, Jan. 2009, doi: 10.1016/j.clinph.2008.10.001.
[18] M. P. Tramonti Fantozzi et al., ‘Trigeminal Stimulation and Visuospatial Performance: The Struggle between Chewing and Trigeminal Asymmetries’, Biomedicines, vol. 11, no. 8, p. 2307, Aug. 2023, doi: 10.3390/biomedicines11082307.
[19] M. P. Tramonti Fantozzi et al., ‘Chewing and Cognitive Improvement: The Side Matters’, Front. Syst. Neurosci., vol.
15, Dec. 2021, doi: 10.3389/fnsys.2021.749444.
[20] K. J. Ferro et al., ‘THE GLOSSARY OF PROSTHODONTIC TERMS Ninth Edition Editorial Staff Glossary of Prosthodontic Terms Committee of the Academy of Prosthodontics’.
[21] B. Pjetursson, A. Asgeirsson, M. Zwahlen, and I. Sailer, ‘Improvements in implant dentistry over the last decade: comparison of survival and complication rates in older and newer publications’, The International journal of oral & maxillofacial implants, vol. 29 Suppl, no. Supplement, pp. 308–324, Jan. 2014, doi: 10.11607/JOMI.2014SUPPL.G5.2.
[22] I. Sailer, S. Mühlemann, M. Zwahlen, C. H. F. Hämmerle, and D. Schneider, ‘Cemented and screw-retained implant reconstructions: A systematic review of the survival and complication rates’, Clinical Oral Implants Research, vol. 23, no. SUPPL.6, pp. 163–201, Oct. 2012, doi: 10.1111/j.1600-0501.2012.02538.x.
[23] S. Storelli, M. Del Fabbro, M. Scanferla, G. Palandrani, and E. Romeo, ‘Implant-supported cantilevered fixed dental rehabilitations in fully edentulous patients: Systematic review of the literature. Part II’, Clinical Oral Implants Research, vol. 29, pp. 275–294, Oct. 2018, doi: 10.1111/clr.13310.
[24] J. G. Wittneben, T. Joda, H. P. Weber, and U. Brägger, ‘Screw retained vs. cement retained implant-supported fixed dental prosthesis’, Periodontology 2000, vol. 73, no. 1, pp. 141–151, Feb. 2017, doi: 10.1111/PRD.12168.
[25] L. Ortensi, M. Ortensi, A. Minghelli, and F. Grande, ‘Implant-Supported Prosthetic Therapy of an Edentulous Patient: Clinical and Technical Aspects’, Prosthesis, vol. 2, no. 3, pp. 140–152, Jul. 2020, doi: 10.3390/PROSTHESIS2030013.
[26] A. Piattelli et al., ‘Fluids and Microbial Penetration in the Internal Part of Cement-Retained Versus Screw-Retained Implant-Abutment Connections’, Journal of Periodontology, vol. 72, no. 9, pp. 1146–1150, Sep. 2001, doi: 10.1902/jop.2000.72.9.1146.
[27] S. K. Kim et al., ‘An abutment screw loosening study of a Diamond Like Carbon-coated CP titanium implant’, J Oral Rehabil, vol. 32, no. 5, pp. 346–350, May 2005, doi: 10.1111/j.1365-2842.2004.01475.x.
[28] I. Sailer, D. Karasan, A. Todorovic, M. Ligoutsikou, and B. E. Pjetursson, ‘Prosthetic failures in dental implant therapy’, Periodontol 2000, vol. 88, no. 1, pp. 130–144, Feb. 2022, doi: 10.1111/prd.12416.
[29] B. E. Pjetursson, N. A. Valente, M. Strasding, M. Zwahlen, S. Liu, and I. Sailer, ‘A systematic review of the survival and complication rates of zirconia‐ceramic and metal‐ceramic single crowns’, Clinical Oral Implants Res, vol. 29, no. S16, pp. 199–214, Oct. 2018, doi: 10.1111/clr.13306.
[30] P. Amornvit, ‘Stress Distribution in Implant Retained Finger Prosthesis: A Finite Element Study’, JCDR, 2013, doi: 10.7860/JCDR/2013/7001.3775.
[31] R. Gaddale, S. K. Mishra, and R. Chowdhary, ‘Complications of screw- and cement-retained implant-supported full-arch restorations: a systematic review and meta-analysis’, Int J Oral Implantol (Berl), vol. 13, no. 1, pp. 11–40, 2020.
[32] D. Flanagan, ‘Bite force and dental implant treatment: a short review’, MDER, vol. Volume 10, pp. 141–148, Jun. 2017, doi: 10.2147/MDER.S130314.
[33] W.-Q. Chen, S.-M. Zhang, and J. Qiu, ‘Surface analysis and corrosion behavior of pure titanium under fluoride exposure’, The Journal of Prosthetic Dentistry, vol. 124, no. 2, p. 239.e1-239.e8, Aug. 2020, doi: 10.1016/j.prosdent.2020.02.022.
[34] Y. Yi, K. Koo, F. Schwarz, H. Ben Amara, and S. Heo, ‘Association of prosthetic features and peri‐implantitis: A cross‐sectional study’, J Clin Periodontol, vol. 47, no. 3, pp. 392–403, Mar. 2020, doi: 10.1111/jcpe.13251.
[35] D. Bourgeois, C. Bernard, and U. Lyon, ‘The Global Burden of Oral Diseases and Risks to Oral Health The Oral Microbiome is a Key factor in Oral and Systemic Health View project Oral microbiota View project’, doi: 10.1590/S0042-96862005000900011.
[36] C. J. Goodacre, G. Bernal, K. Rungcharassaeng, and J. Y. K. Kan, ‘Clinical complications with implants and implant prostheses’, The Journal of Prosthetic Dentistry, vol. 90, no. 2, pp. 121–132, Aug. 2003, doi: 10.1016/S0022-3913(03)00212-9.
[37] J. Schmitz, ‘Complicanze meccaniche odontoiatria protesica AIOP’.
[38] P. Palattella, ‘INCIDENTI E COMPLICANZE IN IMPLANTOLOGIA’.
[39] M. Armentia, M. Abasolo, I. Coria, J. Albizuri, and J. Aguirrebeitia, ‘Fatigue performance of prosthetic screws used in dental implant restorations: Rolled versus cut threads’, The Journal of Prosthetic Dentistry, vol. 126, no. 3, p. 406.e1-406.e8, Sep. 2021, doi: 10.1016/j.prosdent.2021.06.035.
[40] G. Priest, J. Smith, and M. G. Wilson, ‘Implant survival and prosthetic complications of mandibular metal-acrylic resin implant complete fixed dental prostheses’, The Journal of Prosthetic Dentistry, vol. 111, no. 6, pp. 466–475, Jun. 2014, doi: 10.1016/j.prosdent.2013.07.027.
[41] M. C. Pozzan, F. Grande, E. Mochi Zamperoli, F. Tesini, M. Carossa, and S. Catapano, ‘Assessment of Preload Loss after Cyclic Loading in the OT Bridge System in an “All-on-Four” Rehabilitation Model in the Absence of One and Two Prosthesis Screws’, Materials, vol. 15, no. 4, p. 1582, Feb. 2022, doi: 10.3390/ma15041582.
[42] F. Grande, P. M. Cesare, E. Mochi Zamperoli, C. M. Gianoli, F. Mollica, and S. Catapano, ‘Evaluation of Tension and Deformation in a Mandibular Toronto Bridge Anchored on Three Fixtures Using Different Framework Materials, Abutment Systems, and Loading Conditions: A FEM Analysis’, Eur J Dent, p. s-0042-1758785, Jan. 2023, doi: 10.1055/s-0042-1758785.
[43] F. Grande, A. Acquadro, M. C. Pozzan, and S. Catapano, ‘Fattori influenzanti la perdita di precarico delle viti protesiche: una revisione critica’, Dental Cadmos, vol. 91, no. 04, p. 265, Mar. 2023, doi: 10.19256/d.cadmos.04.2023.04.
[44] S. Gupta, H. Gupta, and A. Tandan, ‘Technical complications of implant-causes and management: A comprehensive review’, National Journal of Maxillofacial Surgery, vol. 6, no. 1, p. 3, Jan. 2015, doi: 10.4103/0975-5950.168233.
[45] R. Sadid-Zadeh, A. Kutkut, and H. Kim, ‘Prosthetic Failure in Implant Dentistry’, Dental Clinics of North America, vol. 59, no. 1, pp. 195–214, Jan. 2015, doi: 10.1016/j.cden.2014.08.008.
[46] O. Eraslan, O. Inan, and A. Secilmis, ‘The Effect of Framework Design on Stress Distribution in Implant-Supported FPDs: A 3-D FEM Study’, Eur J Dent, vol. 04, no. 04, pp. 374–382, Oct. 2010, doi: 10.1055/s-0039-1697856.
[47] S. J. Riedy, B. R. Lang, and B. E. Lang, ‘Fit of implant frameworks fabricated by different techniques’, The Journal of Prosthetic Dentistry, vol. 78, no. 6, pp. 596–604, Dec. 1997, doi: 10.1016/S0022-3913(97)70011-8.
[48] R. Castillo-Oyagüe, C. D. Lynch, A. S. Turrión, J. F. López-Lozano, D. Torres-Lagares, and M.-J. Suárez-García, ‘Misfit and microleakage of implant-supported crown copings obtained by laser sintering and casting techniques, luted with glass-ionomer, resin cements and acrylic/urethane-based agents’, Journal of Dentistry, vol. 41, no. 1, pp. 90–96, Jan. 2013, doi: 10.1016/j.jdent.2012.09.014.
[49] D. G. de França, M. H. Morais, F. D. das Neves, A. F. Carreiro, and G. A. Barbosa, ‘Precision Fit of Screw- Retained Implant-Supported Fixed Dental Prostheses Fabricated by CAD/CAM, Copy-Milling, and Conventional Methods’, Int J Oral Maxillofac Implants, vol. 32, no. 3, pp. 507–513, Jun. 2017, doi: 10.11607/jomi.5023.
[50] T. Takahashi and J. Gunne, ‘Fit of implant frameworks: An in vitro comparison between two fabrication techniques’, The Journal of Prosthetic Dentistry, vol. 89, no. 3, pp. 256–260, Mar. 2003, doi: 10.1067/mpr.2003.40.
[51] E. T. Akçin, M. B. Güncü, G. Aktaş, and Y. Aslan, ‘Effect of manufacturing techniques on the marginal and internal fit of cobalt-chromium implant-supported multiunit frameworks’, The Journal of Prosthetic Dentistry, vol. 120, no. 5, pp. 715–720, Nov. 2018, doi: 10.1016/j.prosdent.2018.02.012.
[52] S.-B. Kim, N.-H. Kim, J.-H. Kim, and H.-S. Moon, ‘Evaluation of the fit of metal copings fabricated using stereolithography’, J Prosthet Dent, vol. 120, no. 5, pp. 693–698, Nov. 2018, doi: 10.1016/j.prosdent.2018.01.012.
[53] G. Bayramoglu, T. Alemdaroglu, S. Kedici, and A. A. Aksut, ‘The effect of pH on the corrosion of dental metal alloys’, J Oral Rehabil, vol. 27, no. 7, pp. 563–575, Jul. 2000, doi: 10.1046/j.1365-2842.2000.00549.x.
[54] M. Bergman, ‘Corrosion in the oral cavity–potential local and systemic effects’, Int Dent J, vol. 36, no. 1, pp. 41–44, Mar. 1986.
[55] C. Molina et al., ‘Dental casting alloys behaviour during power toothbrushing with toothpastes of various abrasivities. Part II: corrosion and ion release’, J Mater Sci: Mater Med, vol. 19, no. 9, pp. 3015–3019, Sep. 2008, doi: 10.1007/s10856-008-3432-3.
[56] J. F. McCabe and A. Walls, Applied dental materials, 9th ed. Oxford, UK ; Ames, Iowa: Blackwell Pub, 2008.
[57] K.-T. Oh and K.-N. Kim, ‘Electrochemical properties of suprastructures galvanically coupled to a titanium implant’, J Biomed Mater Res B Appl Biomater, vol. 70, no. 2, pp. 318–331, Aug. 2004, doi: 10.1002/jbm.b.30046.
[58] H. Serhan, ‘Is galvanic corrosion between titanium alloy and stainless steel spinal implants a clinical concern?*1’, The Spine Journal, vol. 4, no. 4, pp. 379–387, Aug. 2004, doi: 10.1016/j.spinee.2003.12.004.
[59] S. H. Tuna, N. O. Pekmez, F. Keyf, and F. Canli, ‘The electrochemical properties of four dental casting suprastructure alloys coupled with titanium implants’, J Appl Oral Sci, vol. 17, no. 5, pp. 467–475, 2009, doi: 10.1590/s1678-77572009000500022.
[60] A. P. G. Jaime, D. K. de Vasconcellos, A. M. M. Mesquita, E. T. Kimpara, and M. A. Bottino, ‘Effect of cast rectifiers on the marginal fit of UCLA abutments’, J. Appl. Oral Sci., vol. 15, no. 3, pp. 169–174, Jun. 2007, doi: 10.1590/S1678-77572007000300004.
[61] R. Galo, R. F. Ribeiro, R. C. S. Rodrigues, L. A. Rocha, and M. da G. C. de Mattos, ‘Effects of chemical composition on the corrosion of dental alloys’, Braz. Dent. J., vol. 23, no. 2, pp. 141–148, Apr. 2012, doi: 10.1590/S0103-64402012000200009.
[62] S. P. Kedici, A. A. Aksüt, M. A. Kílíçarslan, G. Bayramog Lu, and K. Gökdemir, ‘Corrosion behaviour of dental metals and alloys in different media: CORROSION OF DENTAL METALS AND ALLOYS’, Journal of Oral Rehabilitation, vol. 25, no. 10, pp. 800–808, Oct. 1998, doi: 10.1046/j.1365-2842.1998.00305.x.
[63] L. Reclaru, H. Lüthy, P.-Y. Eschler, A. Blatter, and C. Susz, ‘Corrosion behaviour of cobalt–chromium dental alloys doped with precious metals’, Biomaterials, vol. 26, no. 21, pp. 4358–4365, Jul. 2005, doi: 10.1016/j.biomaterials.2004.11.018.
[64] J. C. Wataha, ‘Biocompatibility of dental casting alloys: A review’, The Journal of Prosthetic Dentistry, vol. 83, no. 2, pp. 223–234, Feb. 2000, doi: 10.1016/S0022-3913(00)80016-5.
[65] U. Croatto, V. Moret, and N. Siliprandi, Chimica Generale Con Nozioni di Inorganica, 3a ed. Padova: La Photograph, 1979.
[66] L. Reclaru and J.-M. Meyer, ‘Study of corrosion between a titanium implant and dental alloys’, Journal of Dentistry, vol. 22, no. 3, pp. 159–168, Jun. 1994, doi: 10.1016/0300-5712(94)90200-3.
[67] F. M. S. Soares, C. N. Elias, E. S. Monteiro, M. E. R. Coimbra, and A. I. C. Santana, ‘Galvanic Corrosion of Ti Dental Implants Coupled to CoCrMo Prosthetic Component’, International Journal of Biomaterials, vol. 2021, pp. 1–11, Oct. 2021, doi: 10.1155/2021/1313343.
Ci impegniamo a rispettare la tua privacy. Lavoriamo ogni giorno per ridurre al minimo l’uso dei cookie, anche quelli tecnici, ma alcuni sono indispensabili per permetterci alcune attività. Puoi decidere tu come proseguire.